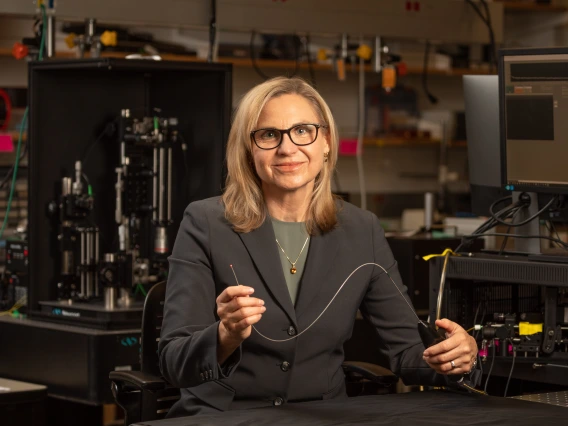
Jennifer Barton wins $3M for tiny microscope to detect endometriosis
The minimally invasive device examines fallopian tubes to aid in early diagnostics and hasten treatment.
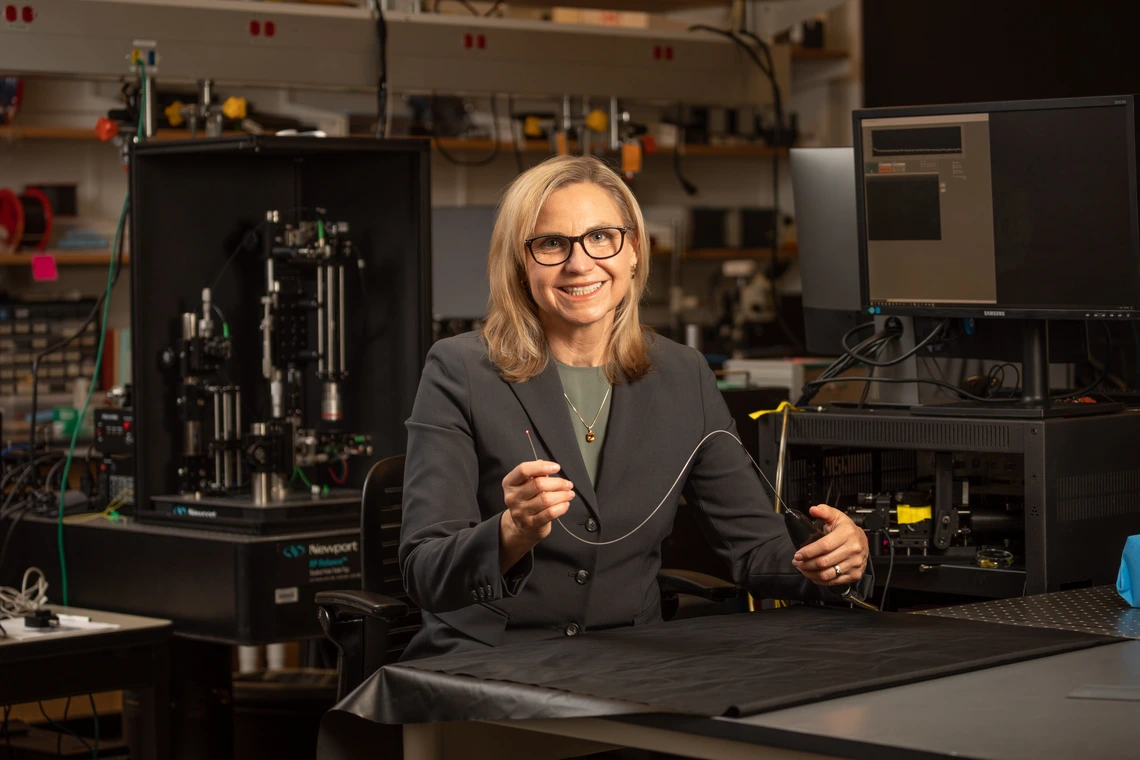
Next up for Jennifer Barton, who is building on her ovarian cancer detection falloposcope, is a microscopic imaging device for endometriosis, one of the leading causes of infertility.
Doctors struggle to diagnose endometriosis, a leading cause of infertility. This painful condition, which often goes undetected for years, affects more than 10% of women ages 15 to 44, according to the Office on Women's Health, an office in the U.S. Department of Health and Human Services.
Jennifer Barton, who holds the Thomas R. Brown Distinguished Chair of Biomedical Engineering, is using a five-year, $3 million award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development to develop a microscopic imaging tool for detecting endometriosis.
Endometriosis is a condition in which tissue similar to the uterine lining grows outside the uterus in places it does not belong, such as the fallopian tubes, the pathway for eggs and embryos. Damaged fallopian tubes can prevent egg and sperm from meeting, a fertilized egg from reaching the uterus, and lead to ectopic pregnancies.
“The fallopian tubes are one of those organs that nobody knows much about,” said Barton, also interim vice provost for health programs at the University of Arizona and member of the BIO5 Institute. “Yet, they are where conception takes place, and they are very important for female reproductive health.”
Seeing inside narrow, squiggly tubes
Doctors need advanced tools to see what’s happening inside the narrow structures.
“They are not just straight tubes,” Barton said. “Inside they have folds, called plicae, that pile up on each other.”
Barton, who holds joint appointments in the Department of Electrical and Computer Engineering and the Wyant College of Optical Sciences, has spent 20 years developing tiny imaging devices that see inside the body.
“Her multidisciplinary approach and innovative technology improve early disease detection, which will be essential to the future of female reproductive health,” said Mario Romero-Ortega, head of the Department of Biomedical Engineering in the College of Engineering.
As the researchers move a patented falloposcope for detecting ovarian cancer – referred to as the silent killer because it is so difficult to catch – through clinical trials and toward adoption, they are using similar technologies for early detection of endometriosis.
The team is building a 1 mm-wide scope – about the size of the tip of a sewing needle – to navigate the tiny fallopian tubes. The device uses optical coherence tomography, near-infrared imaging, to penetrate tissue and capture hundreds of images per second of cilia – hairlike tendrils that line the organs and aid in transport.
“Cilia are very important for keeping organs clean and for moving contents along, but they’re microscopic,” said Barton.
Healthy cilia beat in coordinated, wave-like patterns, but when endometriosis damages fallopian tubes, the cilia movement becomes erratic.
For earlier and more accurate diagnoses of endometriosis, Barton’s team is using the device to identify how cilia beat out of sync in damaged fallopian tubes.
MD-PhD candidate linchpin for study
“The people who really do the work are my students,” said Barton.
Dilara Long, a biomedical engineering doctoral student and MD-PhD candidate in the College of Medicine – Tucson, has generated data that shows OCT can precisely measure the frequency of cilia beating in human tissue samples.
The study, “Optical Coherence Tomography Enables the Depth-Resolved Measurement of Cilia Beat Frequency in Ex Vivo Human Fallopian Tubes,” was published in July 2025 in Lasers in Surgery and Medicine, the journal of the American Society for Laser Medicine and Surgery Inc.
“Despite the important role of cilia in reproduction, little is known about how they function in the human fallopian tubes,” said Long. “We have shown that optical coherence tomography imaging can reveal the location and beat frequency of surface and hidden fallopian tube cilia, potentially advancing understanding, diagnosis and management of reproductive disorders.”
Applications beyond endometriosis
Now the challenge becomes designing a precise exam tool that can separate cilia motions from the movement of the scope and patient.
Barton’s team is applying machine learning algorithms to isolate and analyze cilia activity and partnering with Baylor College of Medicine to broaden the study's reach and ensure diverse patient participation.
Beyond endometriosis detection, she envisions the tool helping doctors address various fertility issues and other reproductive diseases linked to cilia function.
"Other female reproductive infections and diseases will affect cilia," she said. "Understanding whether an infertility issue is tubal, for example, would help determine the next course of action."